![A) Positive-ion APCI-QTOF-MS2 spectra of the [M − H2O + H]+ ions at m/z... | Download Scientific Diagram A) Positive-ion APCI-QTOF-MS2 spectra of the [M − H2O + H]+ ions at m/z... | Download Scientific Diagram](https://www.researchgate.net/publication/224897211/figure/fig1/AS:271220616724496@1441675442264/A-Positive-ion-APCI-QTOF-MS2-spectra-of-the-M-H2O-H-ions-at-m-z-162076-generated.png)
A) Positive-ion APCI-QTOF-MS2 spectra of the [M − H2O + H]+ ions at m/z... | Download Scientific Diagram

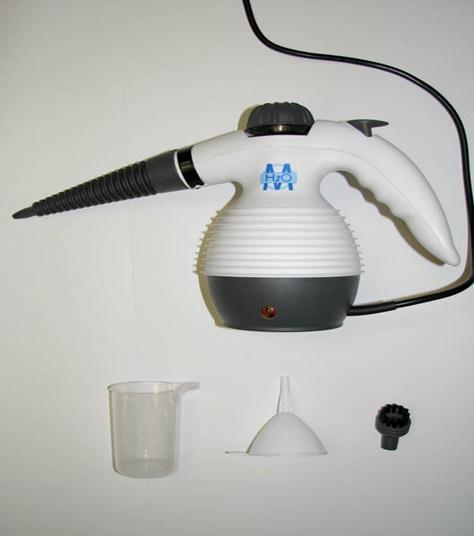
H2O Mop X5 Basic Mop 5 in 1 All Purpose Hand Held Steam Cleaner for Home Use, with 11 Piece Accessory Kit

Consider the following reaction and its equilibrium constant: 4 CuO(s) + CH4(g) ⇌ CO2(g) + 4 Cu(s) + - Brainly.com
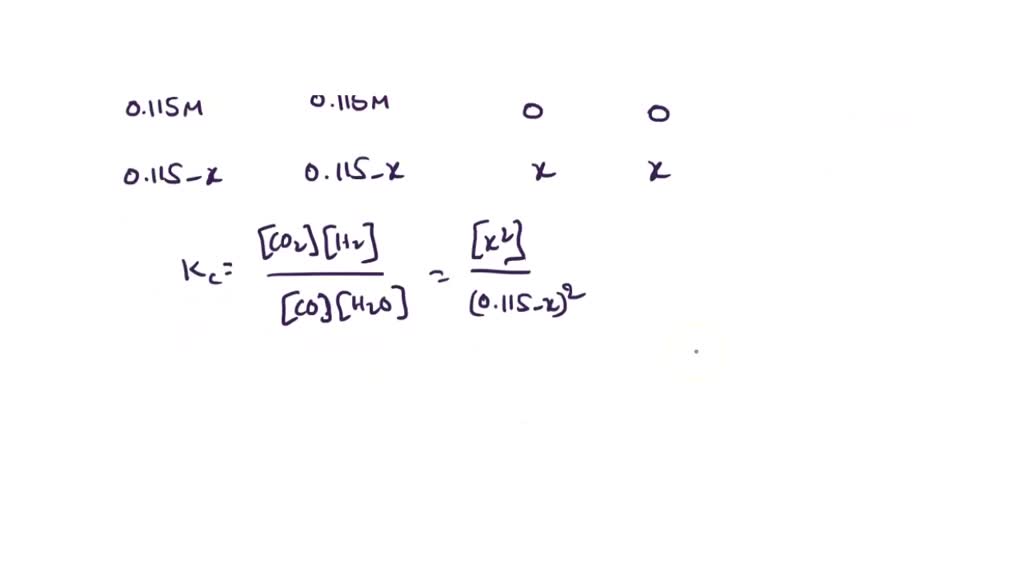
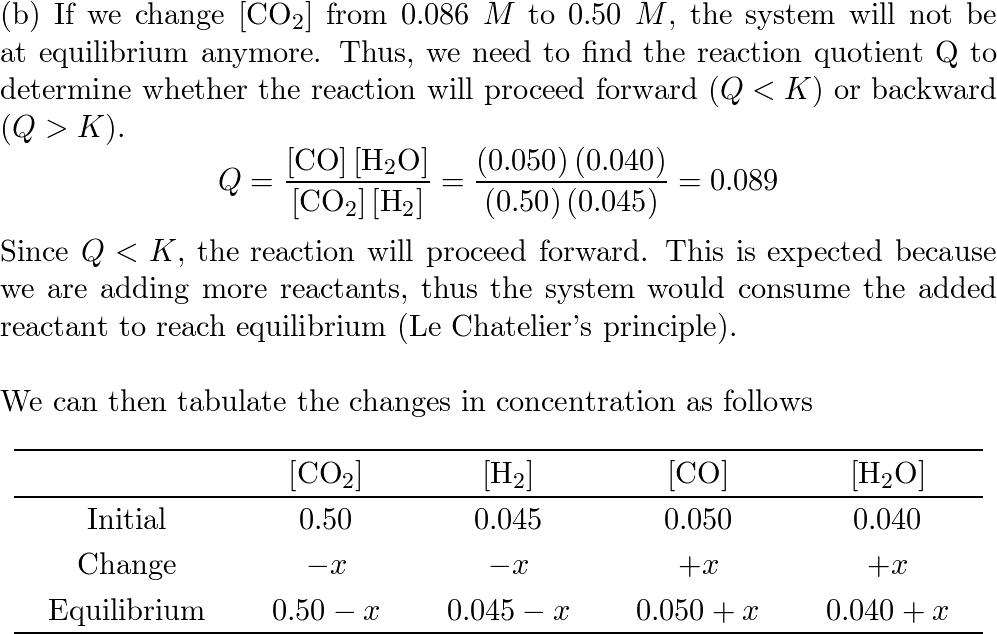
SOLVED: Consider the reaction CO(g)+H2O(g)⇌CO2(g)+H2(g) Kc=102 at 500 K A reaction mixture initially contains 0.115 MCO and 0.115 M H2O. Part A What will be the equilibrium concentration of CO?

Formaldehyde solution, 37 wt. % in H2O, concentration de 13.32 M | 50-00-0 | Reference standards | Alsachim

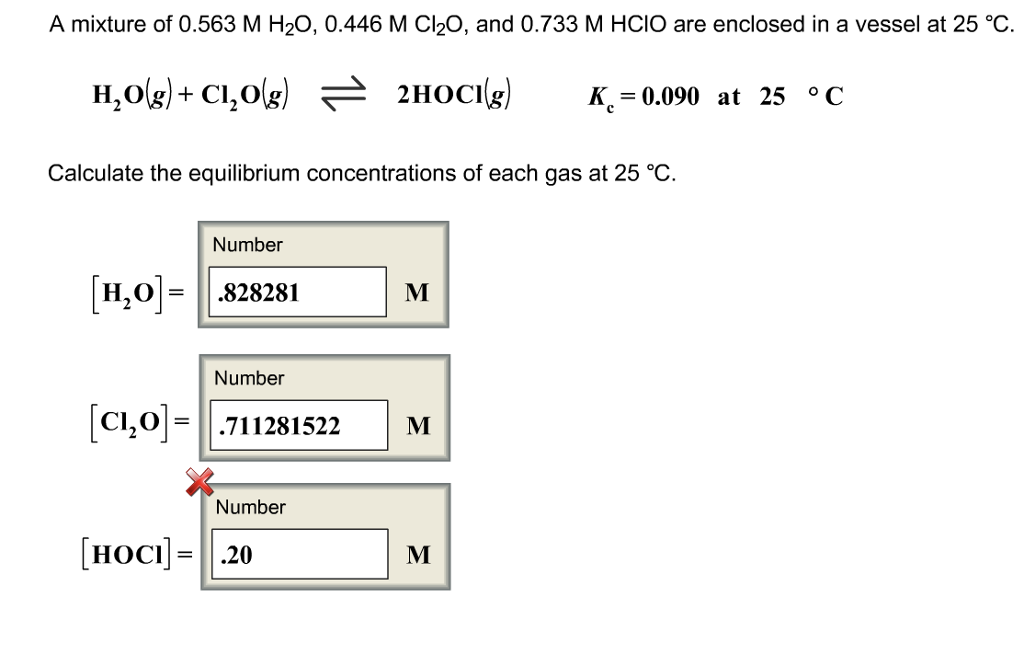
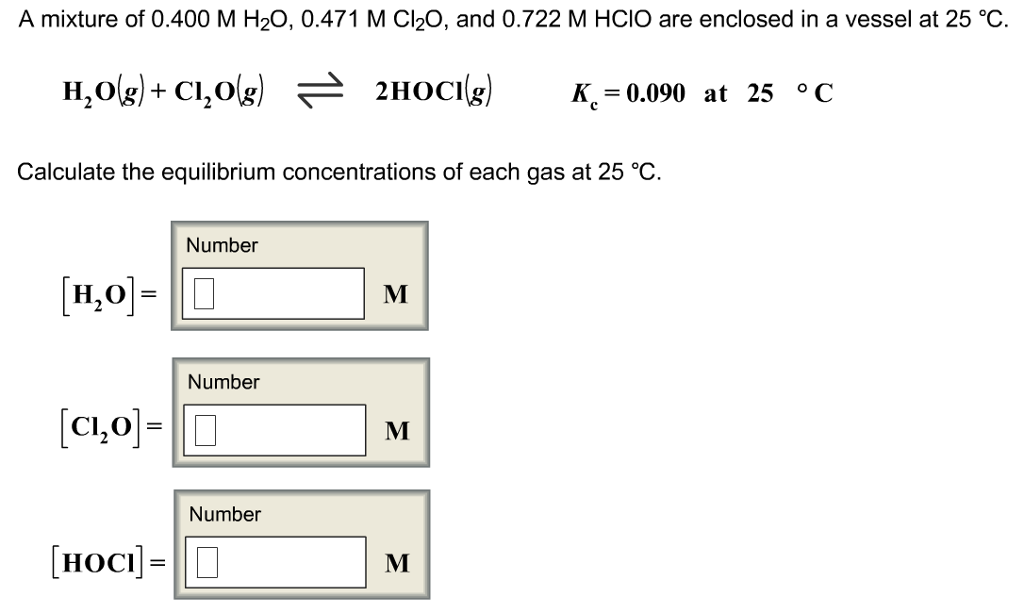
SOLVED: A mixture of 0.466 M H2O , 0.481 M Cl2O , and 0.718 M HClO are enclosed in a vessel at 25°C . H2O(g)+Cl2O(g)↽−−⇀2HOCl(g)𝐾c=0.0900 at 25°C Calculate the equilibrium concentrations of

![Stálost v roztoku [M(H2O)6] [MLn] [ML(n – 1) ] · [L] k k3 (kn) = - ppt stáhnout Stálost v roztoku [M(H2O)6] [MLn] [ML(n – 1) ] · [L] k k3 (kn) = - ppt stáhnout](https://slideplayer.cz/2479776/8/images/slide_1.jpg)
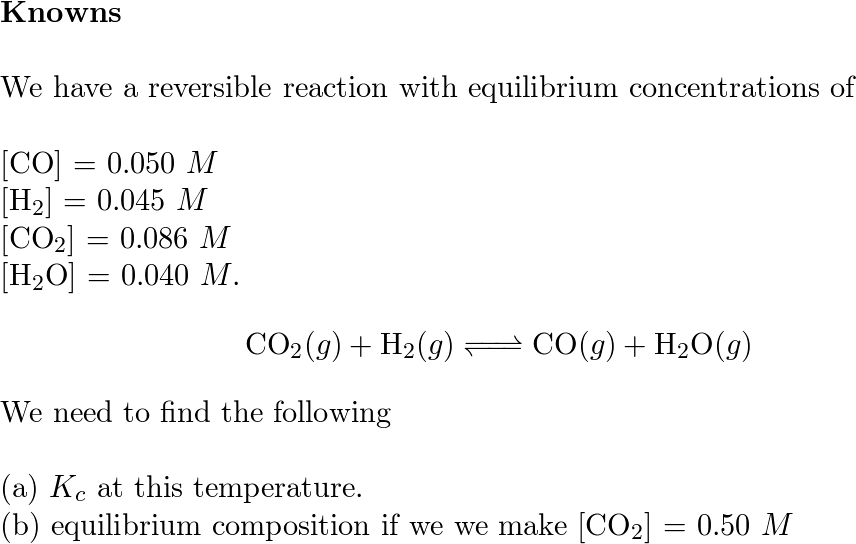



![ANSWERED] XeFn+ mH2O → A(Contain Xe) + Other Produ... - Inorganic Chemistry ANSWERED] XeFn+ mH2O → A(Contain Xe) + Other Produ... - Inorganic Chemistry](https://media.kunduz.com/media/sug-question/raw/68218045-1658822895.7439263.jpeg)