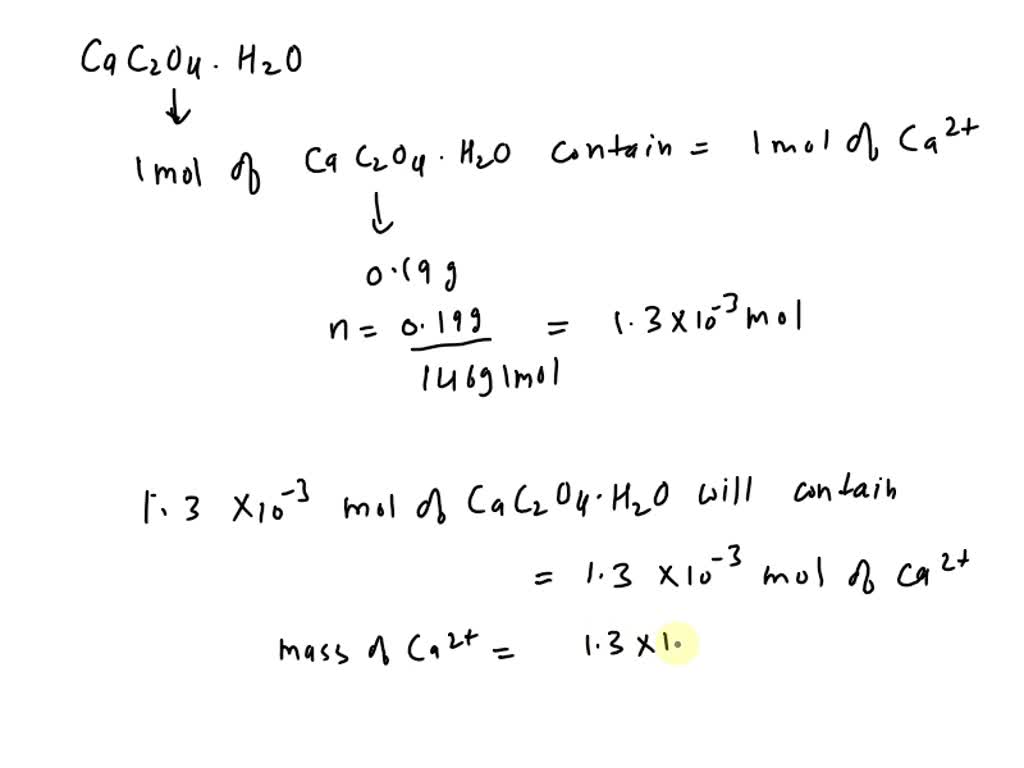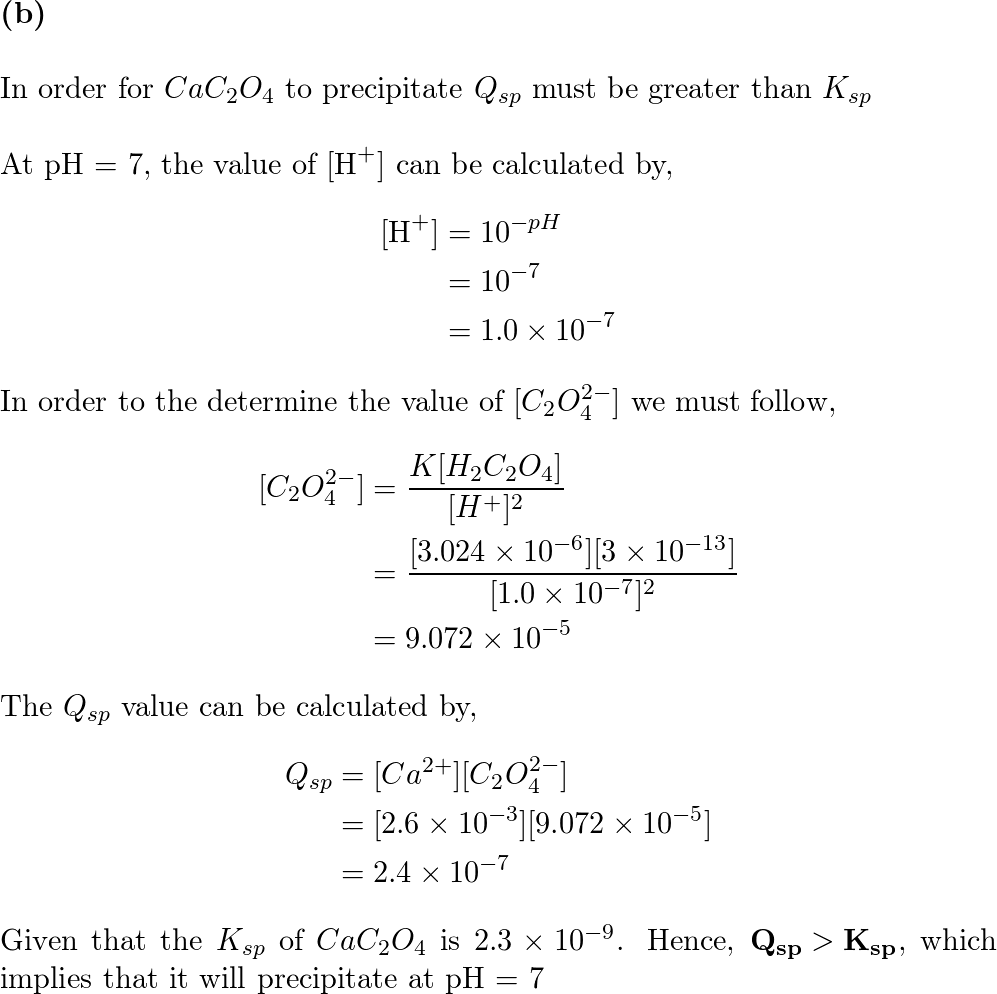A 0.60 g sample consisting of only CaC2O4 and MgC2O4 is heated at 500 ^∘ C , converting the two salts of CaCO3 and MgCO3 . The sample then, weighs 0.465 g .

A 0.60 g sample consisting of only CaC2O4 and MgC2O4 is heated at 500 ^∘ C , converting the two salts of CaCO3 and MgCO3 . The sample then, weighs 0.465 g .
SOLVED: Calcium is determined gravimetrically by precipitating CaC2O4•H2O and isolating CaCO3. After dissolving a sample in 10 mL of water and 15 mL of 6 M HCl, the resulting solution is heated
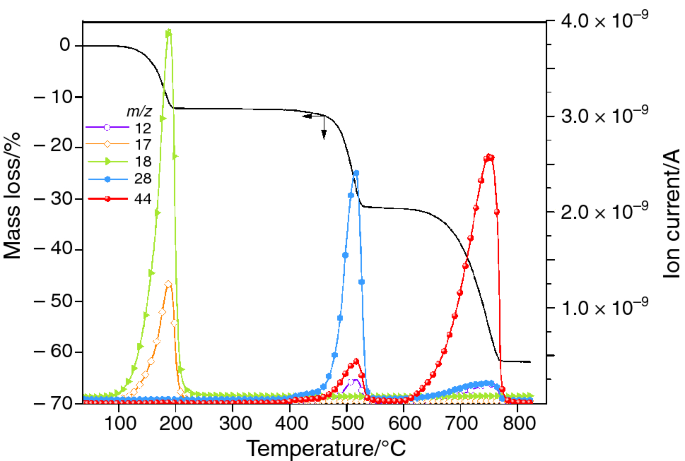
Thermal Stability of Calcium Oxalates from CO2 Sequestration for Storage Purposes: An In-Situ HT-XRPD and TGA Combined Study
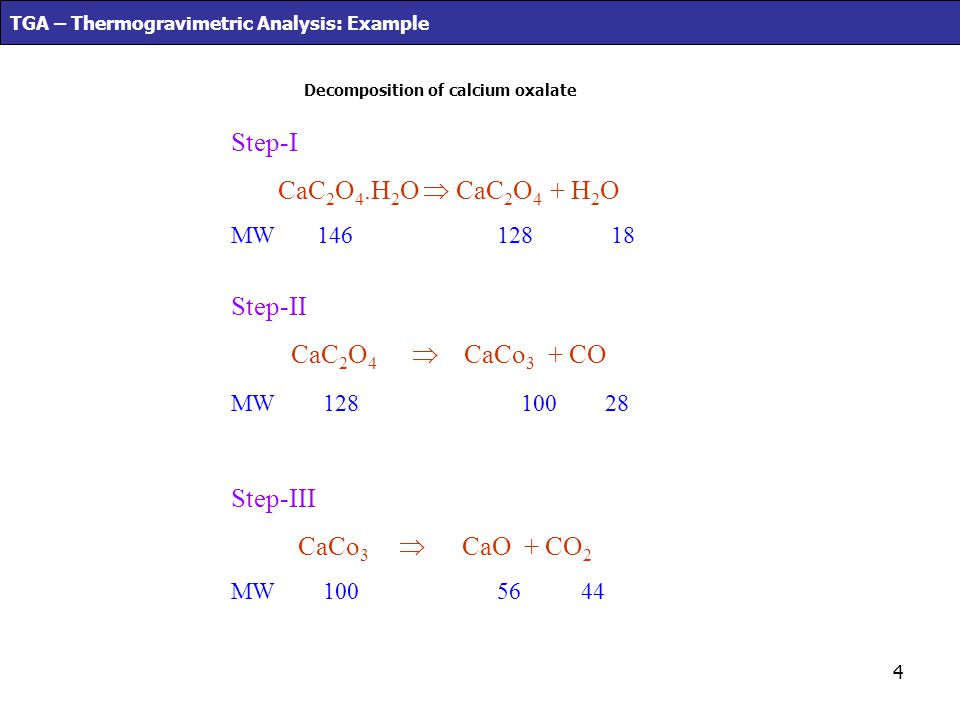
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an
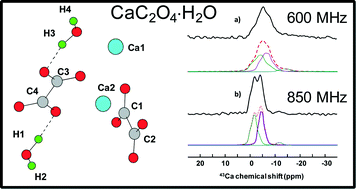
Whewellite, CaC2O4⋅H2O: structural study by a combined NMR, crystallography and modelling approach - CrystEngComm (RSC Publishing)
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an