
Table 2 from H 2 O ) · H 2 O : Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Semantic Scholar
![Give the oxidation state, d - orbital occupation and coordination number of the central metal ion in the following complex. [Mn(H2O)6]SO4 . Give the oxidation state, d - orbital occupation and coordination number of the central metal ion in the following complex. [Mn(H2O)6]SO4 .](https://haygot.s3.amazonaws.com/questions/1873456_1575827_ans_3125df065a114c8e8610e9410b778e2a.jpeg)
Give the oxidation state, d - orbital occupation and coordination number of the central metal ion in the following complex. [Mn(H2O)6]SO4 .
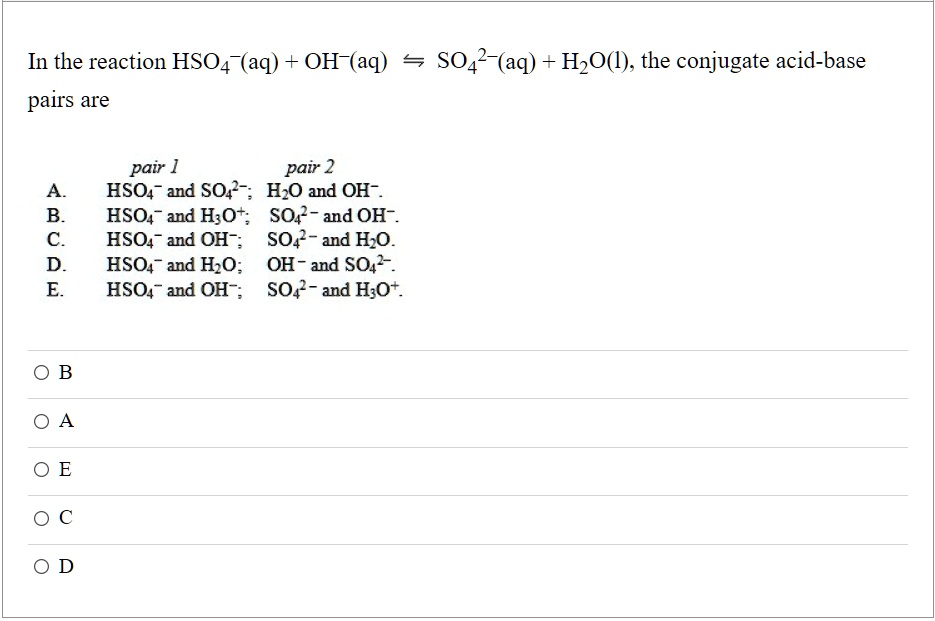
SOLVED: In the reaction HSO4 (aq) + OH-(aq) 5 SO42-(aq) H2O(l), the conjugate acid-base pairs are pair 1 pair 2 HSO- and SO4 -; H,O and OH- HSO4- and H;O;; SO4 -

OneClass: Mixing 39.0g of Al(OH)3 with an excess of H2SO4 we produce Al2(SO4)3. Using the following e...

H2O+H2SO4=H3O+SO4 Balance the equation. h2o+h2so4=h3o+so4 water and Sulfuric acid reacts to form - YouTube

![Thermal effects in [CsEu(H2O)3(SO4)2]·H2O. | Download Scientific Diagram Thermal effects in [CsEu(H2O)3(SO4)2]·H2O. | Download Scientific Diagram](https://www.researchgate.net/profile/Aleksandr-Oreshonkov/publication/354150483/figure/tbl1/AS:1061074447114240@1629991270579/Thermal-effects-in-CsEuH2O3SO42H2O_Q320.jpg)





![Answered: a. CГОЗ HO H,SO4, H2O b. [1] O3 [2] H20… | bartleby Answered: a. CГОЗ HO H,SO4, H2O b. [1] O3 [2] H20… | bartleby](https://content.bartleby.com/qna-images/question/d66f1a49-6a74-4120-82f6-39c04434f2ae/0caae1aa-a18d-4475-b945-b3b8c68a132a/nw3bhka.png)


![Answered: [Ti(H20)6]SO4 has a CFSE of.. a.… | bartleby Answered: [Ti(H20)6]SO4 has a CFSE of.. a.… | bartleby](https://content.bartleby.com/qna-images/question/e071628f-7e30-449a-a238-a5a35c74de5c/3499bed8-ff1b-4740-a320-9481aeb2f93c/n740zs_processed.jpeg)
