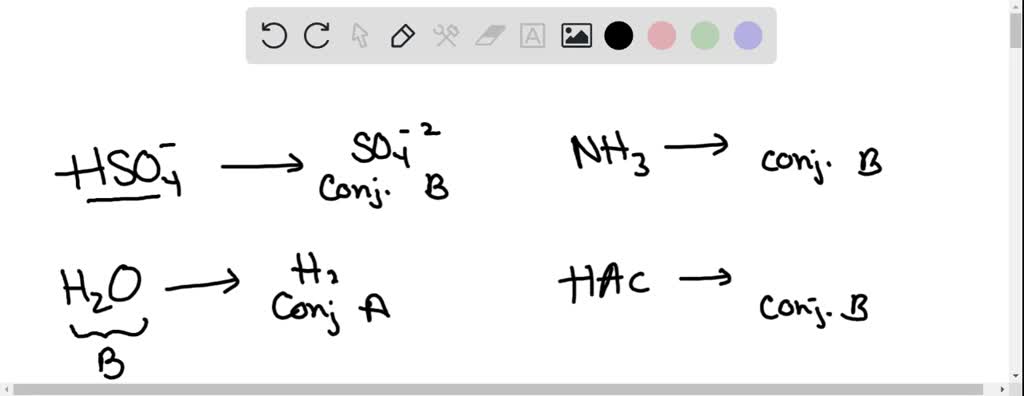
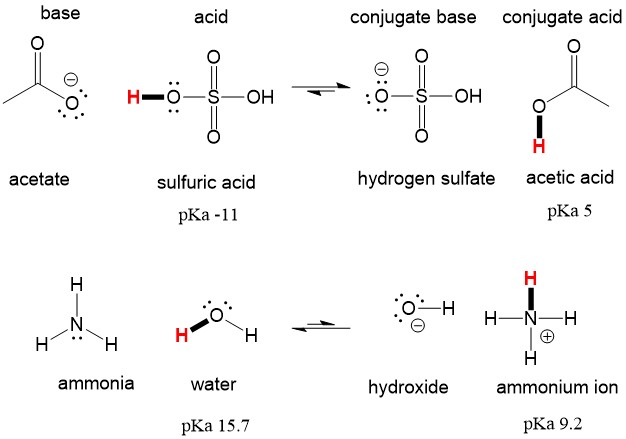
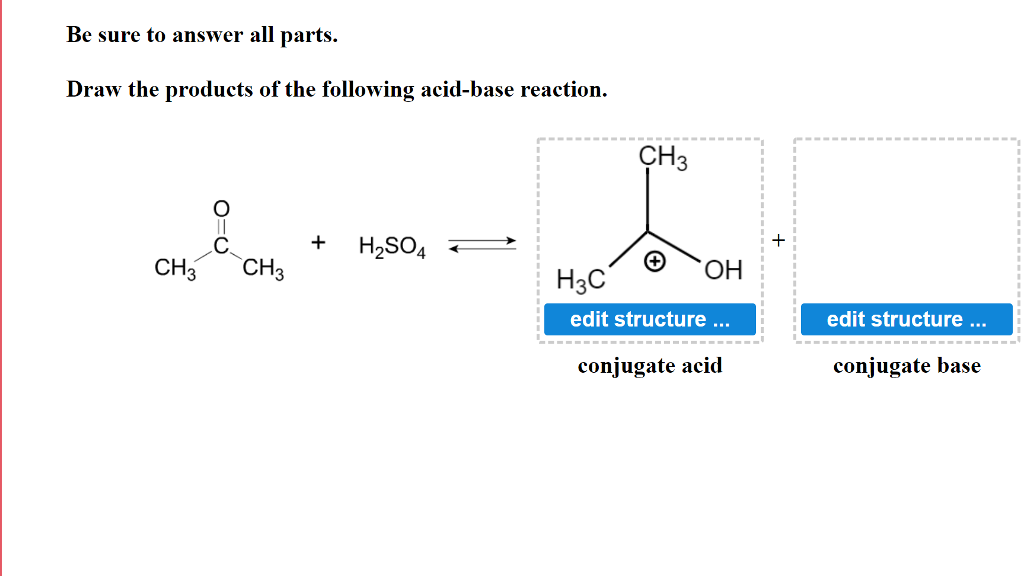
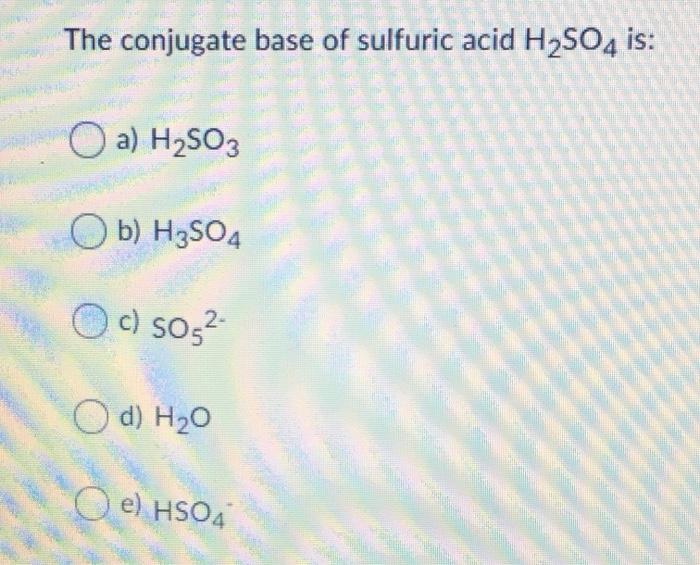
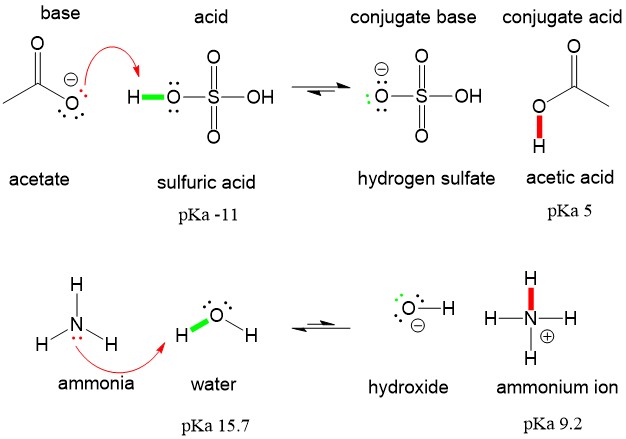
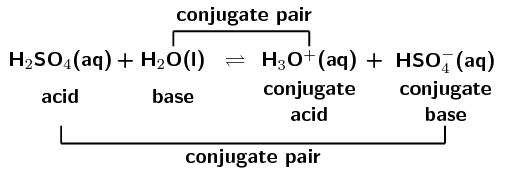
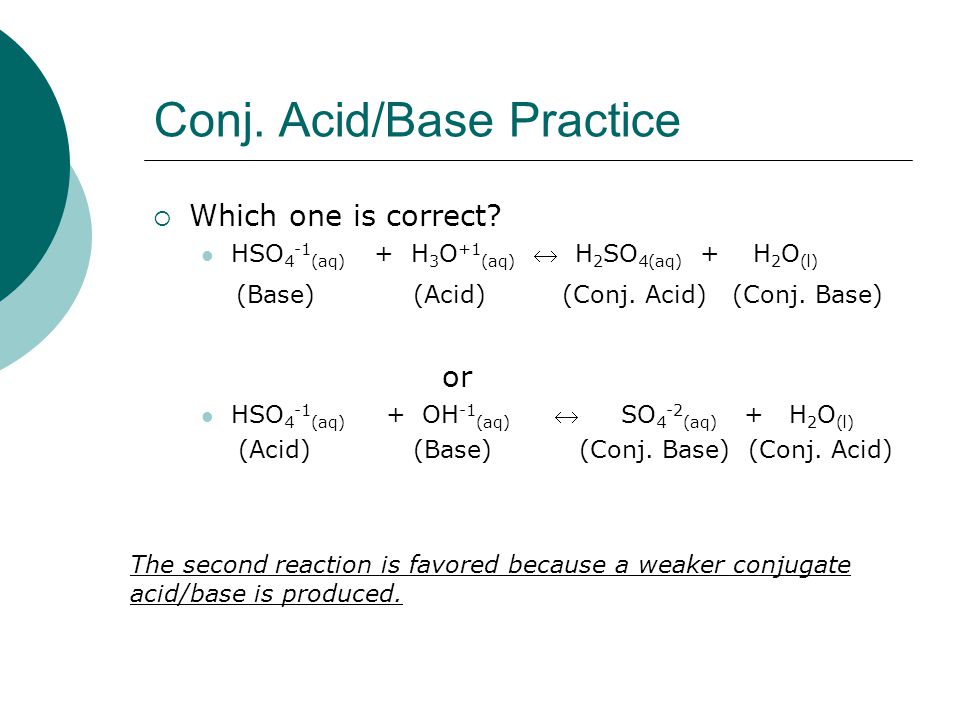
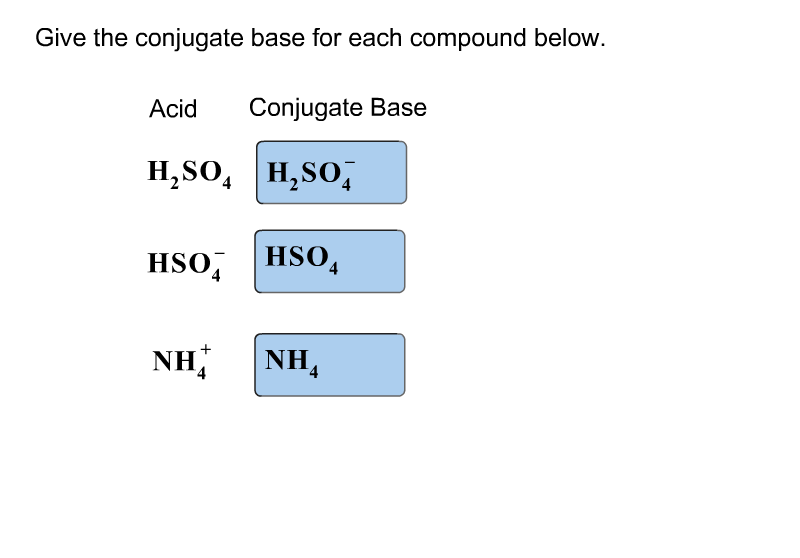
Write a balanced equation for the dissociation of the following in water and identify the conjugate acid - base pairs. (i) NH4^(+) (ii) H2SO4 (iii) CH3COOH

Illustration of the initial acid‐base clustering in urban Beijing. (a)... | Download Scientific Diagram

organic chemistry - Why Does A Brønsted–Lowry Acid Accept Proton from Stronger Acid? - Chemistry Stack Exchange

Identify the acid-conjugate base pair in this balanced equation: H2SO4 + 2NaOH → 2H2O + - Brainly.com