Write the neutralization reaction for the following acid and base: HCl_{(aq)} and KOH_{(aq)}. | Homework.Study.com
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy
Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy
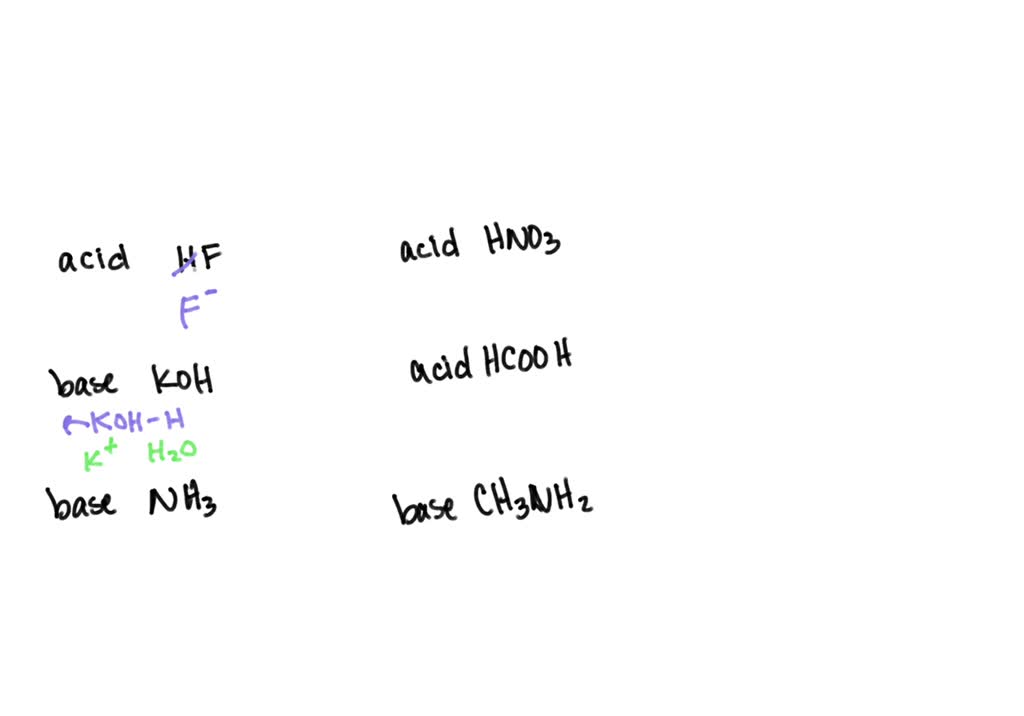
SOLVED: List the conjugate acid or conjugate base for each chemical. a. The acid HF b. The base KOH c. The base NH3 d. The acid HNO3 e. The acid HCOOH f.

SOLVED: Potassium hydroxide dissociates in water to produce hydroxide ions KOH(s) +HzO() Kt(aq) + OH (aq) Given the information above, how is potassium hydroxide categorized? Both a Bronsted-Lowry base ad an Arrhenius
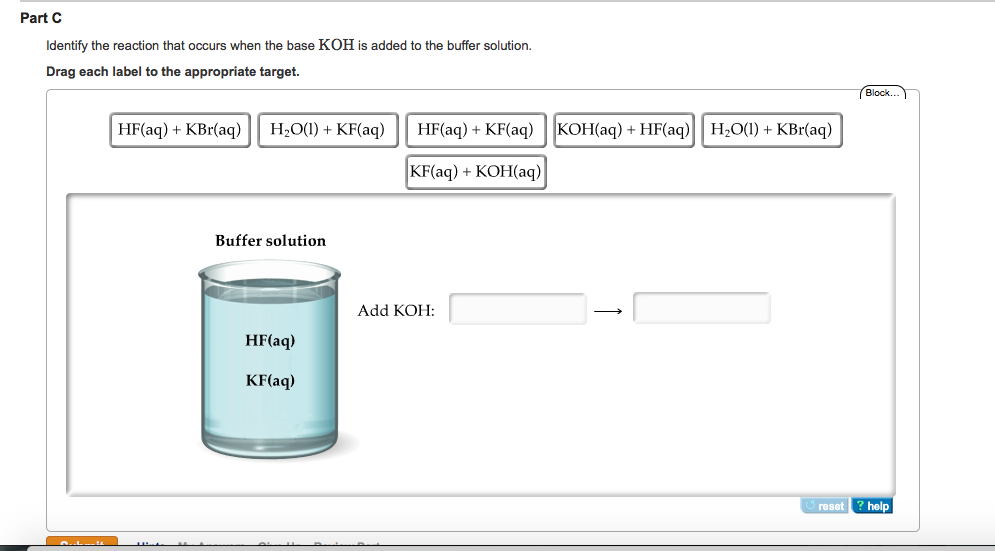
![Identify the reaction that occurs when the base KOH is added to the buffer solution. [{Image src='buffer_solution613505384566154416.jpg' alt='buffer solution' caption=''}] | Homework.Study.com Identify the reaction that occurs when the base KOH is added to the buffer solution. [{Image src='buffer_solution613505384566154416.jpg' alt='buffer solution' caption=''}] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/buffer_solution613505384566154416.jpg)