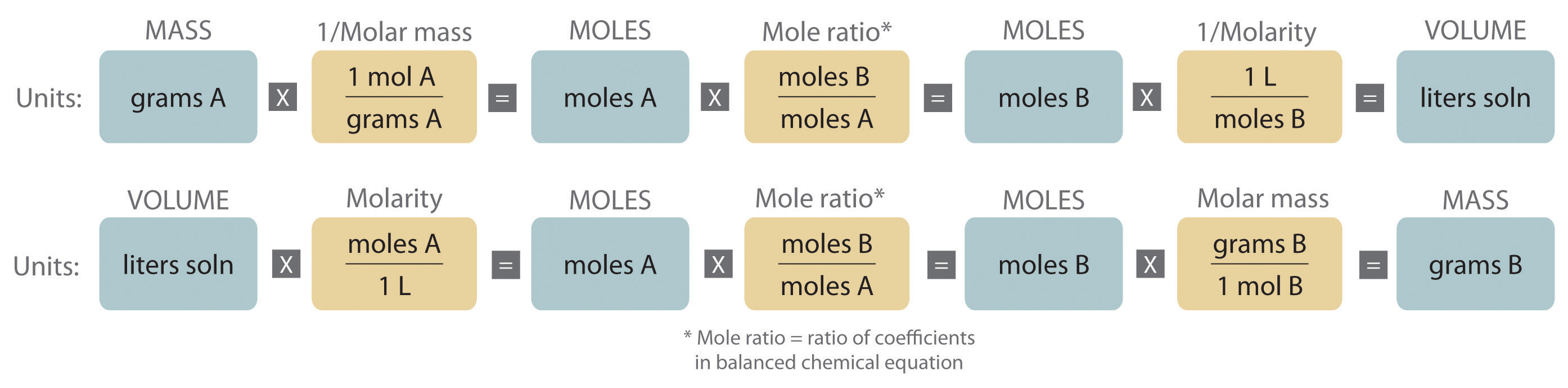
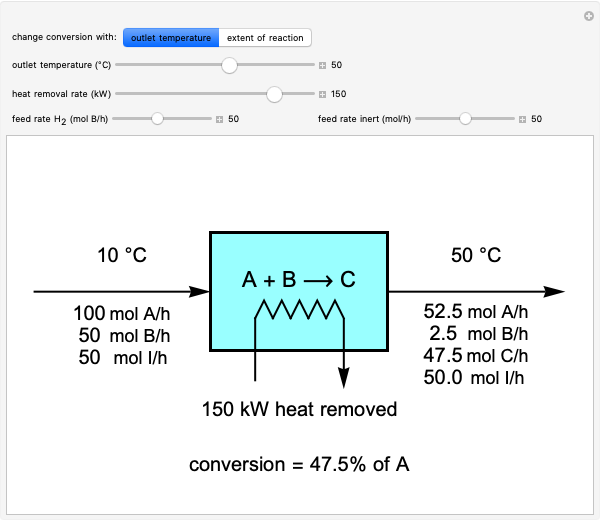
A new definition of the mole has arrived - IUPAC | International Union of Pure and Applied Chemistry

Question Video: Calculating the Mass of Calcium Chloride That Contains a Given Mass of Chlorine | Nagwa

In a reaction A + B2→AB2 , identify the limiting reagent, if any, in the following reaction mixtures.(i) 300 atoms of A + 200 molecules of B2 (ii) 2 mol A +

Computational Mutagenesis at the SARS-CoV-2 Spike Protein/Angiotensin-Converting Enzyme 2 Binding Interface: Comparison with Experimental Evidence | ACS Nano

SOLVED: How many moles of N2O4 are present in 76.3 g of N2O4? The molar mass of N2O4 is 92.02g / mol. A,0.828 B.1.44 x 10^2mol C, 7.02 x 10^3 mol d.
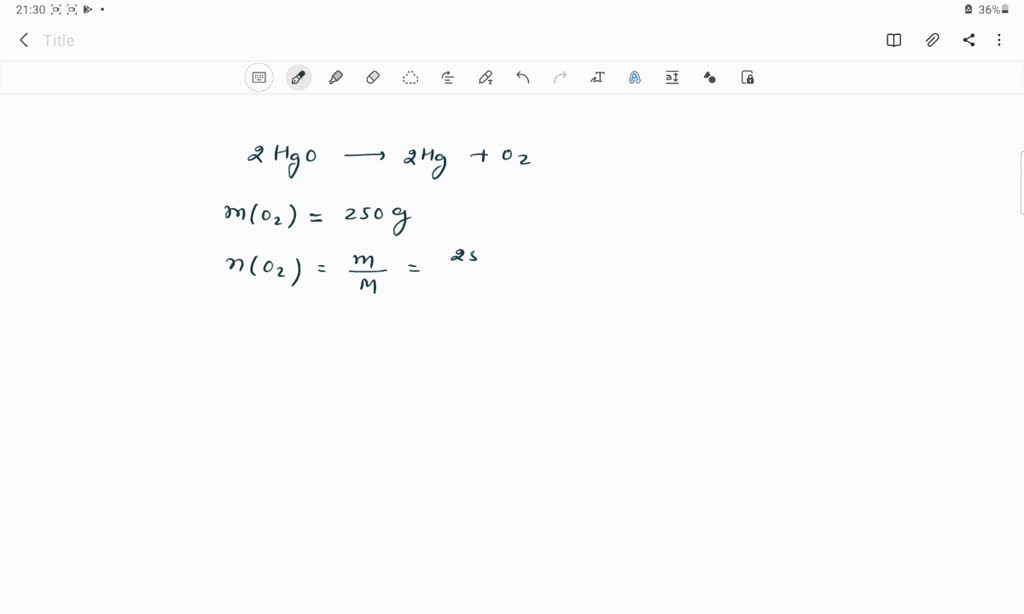
SOLVED: The molar mass of HgO is 216.59 g/mol. The molar mass of O2 is 32.00 g/mol. How many moles of HgO are needed to produce 250.0 g of O2?