How to Balance Na2CO3+H2O→NaOH+CO2 | How to Balance Na2CO3+H2O→NaOH+CO2 | By Organic Chemistry Tutorial/Inorganic Chemistry/Science | Facebook

Chemical reaction-Rusty red iron(III) hydroxide precipitate (Fe(OH)3) in test tube formed reaction between iron(III) chloride (FeCl3) & sodium carbonate (Na2CO3): FeCl3+Na2CO3+H2O ->Fe(OH)3+NaCl+CO2 Stock Photo | Adobe Stock

Phase Equilibria of the NaOH–NaBO2–Na2CO3–H2O System at 30 °C, 60 °C, and 100 °C | Journal of Chemical & Engineering Data
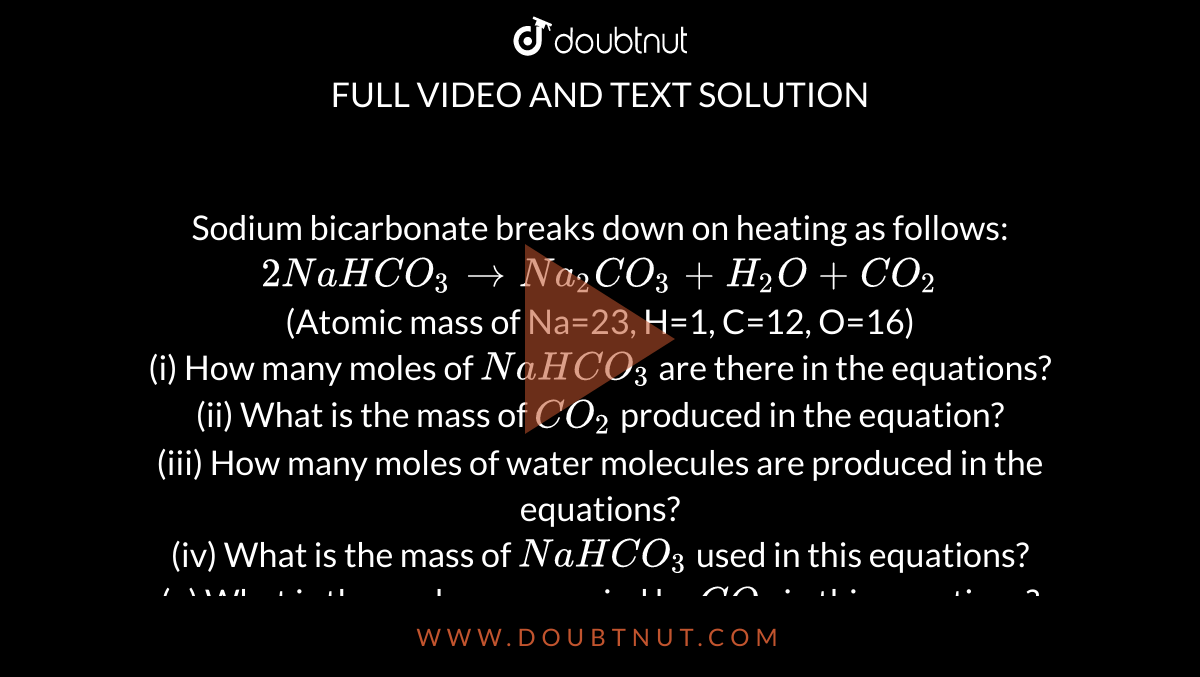
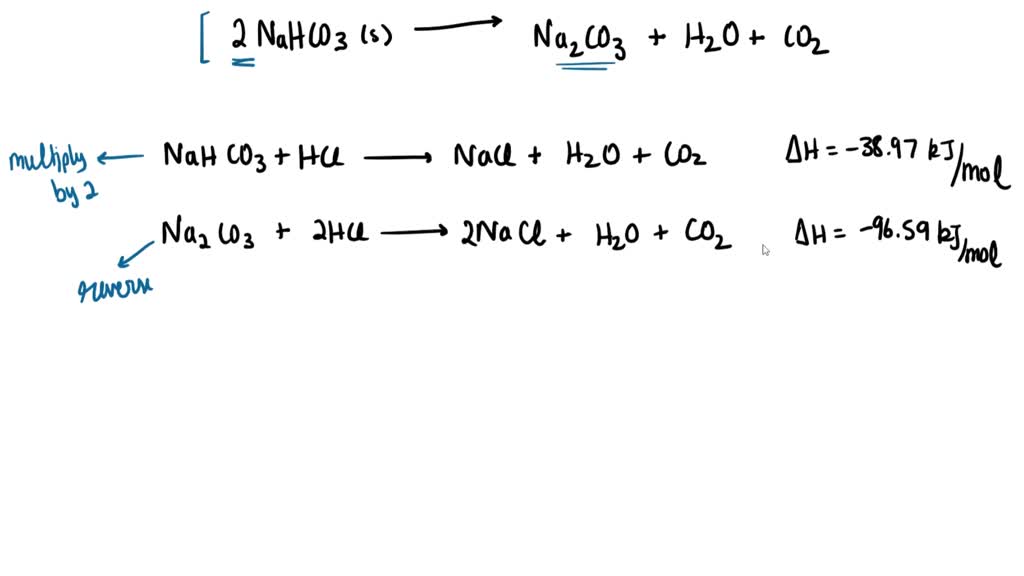
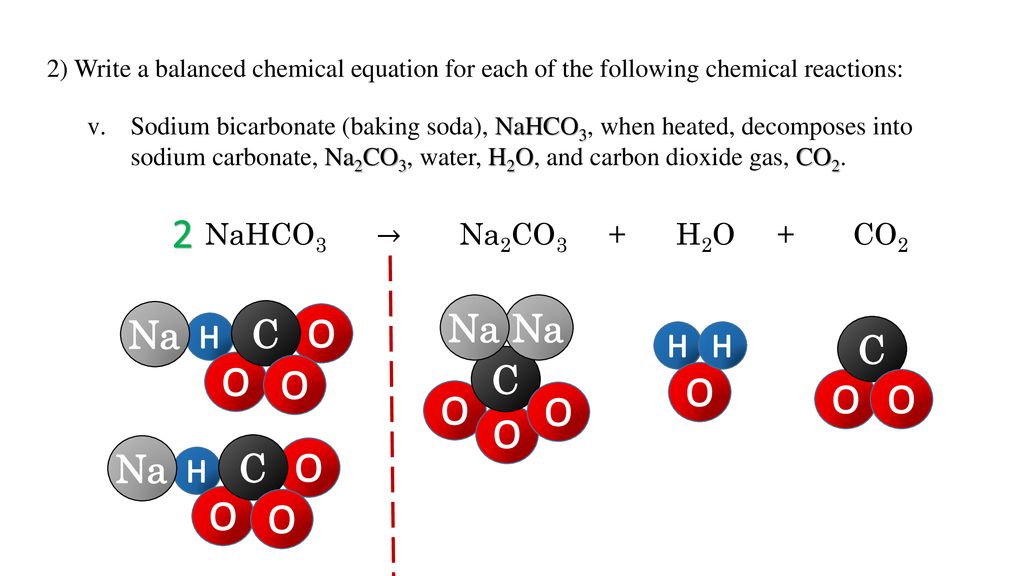
Sodium bicarbonate breaks down on heating as follows: 2NaHCO(3) to Na(2)CO(3)+H(2)O+CO(2) (Atomic mass of Na=23, H=1, C=12, O=16) (i) How many moles of NaHCO(3) are there in the equations? (ii) What is

Synthesis of compounds 4a–t and 6a–j. Reaction conditions and reagents:... | Download Scientific Diagram

Phase Equilibria of the NaOH–NaBO2–Na2CO3–H2O System at 30 °C, 60 °C, and 100 °C | Journal of Chemical & Engineering Data